Nycodenz
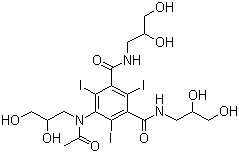
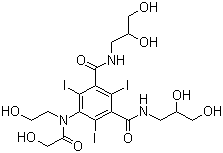
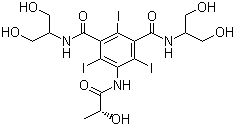
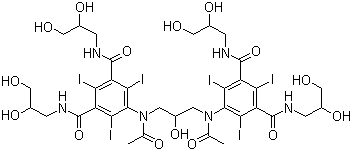
Axis Schield's Nycodenz® is a non-ionic triiodinated derivative of benzoic acid with three
aliphatic hydrophilic side chains.
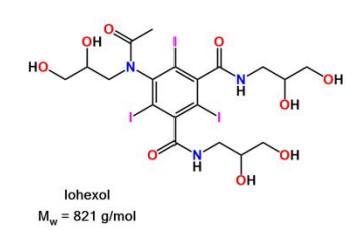
Axis Nycodenz® is the trademark name for Gen's iohexol,
whose systematic name is 5- (N-2, 3-dihydroxypropylacetamido)-2, 4, 6-tri-iodo-N, N'-bis (2, 3 dihydroxypropyl) isophthalamide.
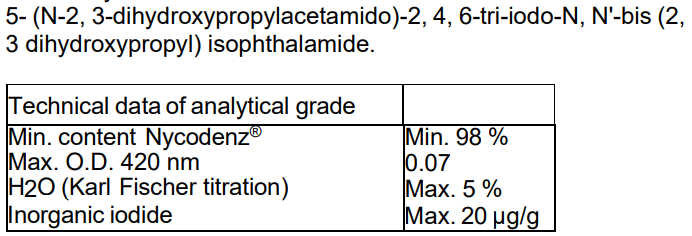
Technical data of analytical grade
Min. content Nycodenz® Min. 98 %
Max. O.D. 420 nm 0.07
H2O (Karl Fischer titration) Max. 5 %
Inorganic iodide Max. 20 µg/g
Nycodenz properties
Axis Shield Nycodenz® has a molecular weight of 821 g/mol and a density of 2.1 g/ml.
This Nycodenz® is non-ionic, non-toxic and is very water soluble. Stock solutions of over 80 % (w/v) can be prepared.
Nycodenz separation medium is also soluble in formamide and dimethyl formamide, thus it is possible to prepare non-aqueous
denaturing gradients of Nycodenz®.
Aqueous solutions of Nycodenz® have a very high water activity. Most particles will therefore be fully hydrated in solutions of Nycodenz® and will
band at a low density. Solutions of Nycodenz® are stable to heat and may be autoclaved, stability to autoclaving is enhanced by the addition of small amounts of Tris and EDTA.
Solutions of Nycodenz® are very resistant to bacterial degradation and Nycodenz® is not metabolized by mammalian cells.
The concentration and density of solutions of Nycodenz® can easily be determined by measuring the refractive index.
Therelationship between concentration, refractive index (n) and density is linear and can be formulated:
- Concentration, % (w/v) = 607.75 n - 810.13
- Density (g/ml) = 3.242 n - 3.323
Before using this equation, the refractive index must be corrected for the presence of buffer or salt in the gradient medium.
Nycodenz® is a non-particulate medium; therefore the distribution of cells in a gradient can be determined using a haemocytometer, electronic particle counter or by light-scattering measurements using a spectrophotometer.

STABILITY AND STORAGE
Nycodenz® in solid form is stable for a period of 5 years when stored at room temperature and protected from light.
Nycodenz® in solution is stable for 5 years provided that it is kept sterile and protected from light. Prolonged exposure to direct sunlight leads
to release of iodine from the molecule.
This effect is negligible when working with these solutions on a day to day basis.
APPLICATIONS
Nycodenz® can be used in the fractionation of nucleic acids, proteins, polysaccharides and nucleoproteins.
Moreover, most types of subcellular organelles can be successfully isolated on gradients of Nycodenz® under either isotonic or mildly hypertonic
conditions. Nycodenz® has a low osmolality, and is non-toxic, thus making it an ideal medium for the separation of intact living
cells.
The low viscosity of Nycodenz® in parallel with it's non-ionic characteristics has proved Nycodenz® to be useful in the isolation
and purification of viruses and bacteria.
FORMATION OF GRADIENTS
Gradients of Nycodenz® can be generated in the following ways:
1. Formed in situ by centrifugation (self-forming
gradients).
2. Layering solutions of the desired concentration into an
appropriate centrifuge tube and allowing the solutions
to diffuse. Using Nycodenz® isotonic solution gradients
can be simply prepared within 45 minutes (see next
section).
3. Freezing and thawing.
4. Gradient mixers.

PREPARATION OF ISOTONIC GRADIENTS OF NYCODENZ®
Gradient solutions for the preparation of essentially iso-osmotic Nycodenz® gradients has been devised and these can be
prepared usinganiso-osmotic solution of Nycodenz®which contains 27.6% (w/v) Nycodenz® (density = 1.15 g/ml) made up in
buffered medium. This solution may be diluted to desired concentration by using a buffered diluent containing either
sucrose or NaCl as osmotic balancer.
The composition of these diluents are as follows:
0.75 g NaCl or 7.45 g sucrose dissolved in 100 ml 5 mmol/I TrisHCI (pH 7.5) containing 3 mmol/l KCI and 0.3 mmol/I
CaNa2EDTA.
The relationship between density and refractive index (n) can
NaCldiluent Sucrose diluent
Density = 3.287 n - 3.383 Density = 3.410 n - 3.555

COMPATABILITY WITH SOME WIDELY USED ASSAYS
Nycodenz® does not interfere with the orcinol and
diphenylamine reactions for estimation of nucleic acids, nor with the very sensitive dyebinding assays for protein and DNA.
Polysaccharides and sugars can be determined in the presence
of Nycodenz® using the phenol/H2SO4 assay.
Fluorimetric assays of nucleic acid and proteins can also be carried out in the
presence of Nycodenz®.
Nycodenz® does not interfere with most assays for the marker enzymes of subcellular components, also most commercial
scintillants arecompatible with Nycodenz®.
REMOVAL OF NYCODENZ® FROM SAMPLES
Nycodenz® can be removed from samples by dialysis, ultrafiltration and gel filtration. Cells, subcellular organelles and other particulate matter can be separated from Nycodenz® by
centrifugation without the risk of contaminating the pellet with Nycodenz®.
Nycodenz® is readily soluble in both acidic and ethanolic
media. Thus, in a number of cases samples can be isolated
free of Nycodenz® by precipitating the sample with
trichloroacetic acid or ethanol.
ORDERING INFORMATION
Nycodenz® prod. no. 18003 1 x 500 g
ISO 13485 certified
References:
-
Equilibrium Density Gradient Centrifugation of the Scrapie Agent in Nycodenz
-
Plasmodium research
-
Apportioning bacterial carbon source utilization in soil using 14C isotope analysis of FISH-targeted bacterial populations sorted by Fluorescence Activated Cell Sorting (FACS): 14C-FISHFACS
-
Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation Rebeca Acin-Perez1, Eric Salazar2, Margarita Kamenetsky3, Jochen Buck3, Lonny R. Levin3, and Giovanni Manfredi1 1 Department of Neurology and Neuroscience, Weill Medical College of Cornell University, New York, NY 10065 2 Tri-Institutional MD-PhD Program, Weill Medical College of Cornell University, New York, NY 10065 3 Department of Pharmacology, Weill Medical College of Cornell University, New York, NY 10065
-
Temozolomide Modifies Caveolin-1 Expression in Experimental Malignant Gliomas In Vitro and In Vivo1 Céline Bruyère*, Laurence Abeloos†, Delphine Lamoral-Theys‡, Rebecca Senetta§, Véronique Mathieu*,2, Marie Le Mercier*,3, Richard E. Kast¶, Paola Cassoni§, Guy Vandenbussche#, Robert Kiss*,2 and Florence Lefranc*,†,2 *Laboratoire de Toxicologie, Faculté de Pharmacie, Université Libre de Bruxelles, Brussels, Belgium; † Service de Neurochirurgie, Hôpital Erasme, Brussels, Belgium; ‡ Laboratoire de Chimie Bioanalytique, Toxicologie et Chimie Physique Appliquée, Faculté de Pharmacie, Université Libre de Bruxelles, Brussels, Belgium; § Department of Biomedical Sciences and Human Oncology, University of Turin, Turin, Italy; ¶ Department of Psychiatry, University of Vermont, Burlington, VT, USA; #Laboratory for the Structure and Function of Biological Membranes, Faculty of Sciences, Université Libre de Bruxelles, Brussels, Belgium